WP6: Health Economy, Commercialisation and Decision Support
As the development activities of DoMore! has progressed and the potential products have advanced in the pipeline, the activities connected to the commercialisation of the projects inventions started during autumn 2018.
The main goal for the first phase was to outline the general strategies for the transfer of technology, rights, and knowhow to a commercial company as well as the principles of ownership of such a company and the revenue streams back to the stakeholders.
 Illustration from the original project application
Illustration from the original project application
Overall strategic commercialisation goal
DoMore!s commercialisation strategy is included in the project’s overall strategy. We believe it will add its value to the project, making it more than a passive owner and seller of IP rights and software components. The base of this strategy is the understanding that in silico pathology rests on the foundation of digital pathology. A “passive” strategy is more vulnerable to the dynamics of the industry, such as a slow distribution of digital pathology. Therefore, a viable strategy includes options for wider involvement in the pathology value chains.
Products and services
A range of artificial intelligence products resulting from the research and development were early identified through the commercialisation discussions. The products in the pipeline in 2018 can be roughly divided into three main groups:
- Pathology workflow optimization
- Prognostic markers
- Screening tools
These three types of products fit in different parts of the value chain of the industry and are thus believed to require different commercial approaches. For some of the products, simple license agreements with hardware vendors may be the optimal commercialisation strategy, whereas for others the optimal strategy for the stock owners may be to develop services or enter into strategic collaborations with existing service providers. Also, planned products within clinical decision support, which is not yet in the pipeline, may require yet another commercialisation strategy.
Patents
DoMore scientists have developed machine learning algorithm trained on some scanner images. Each image is divided into tiles which are then used to train the deep learning algorithm. The trained algorithm may then be used to evaluate images.
A UK patent application covering the methodology described above was filed in the UK on November 16, 2017. A PCT application was filed on November 9, 2018.
The initial company set up
Radforsk and OCC have, on behalf of the parties negotiated the commercial terms, i.e., license/royalty agreements for the first products.
The main element of the company setup was the search for an appointment of CEO which will formally lead the commercialisation activities. The primary task for the CEO will be to develop a business plan and execute this plan to initiate a revenue stream.
Clinical Decision Support System in collaboration with DIPS ASA
As a partner in DoMore! and as a provider of Electronic Patient Journal (EPJ) to the majority of Norwegian hospitals, DIPS is committed to developing an integrated Clinical Decision Support System. The team has started a process based on different deep learning systems to analyze and score prognostic information from DoMore!. The result will be integrated with scores from radiology imaging and clinical information.
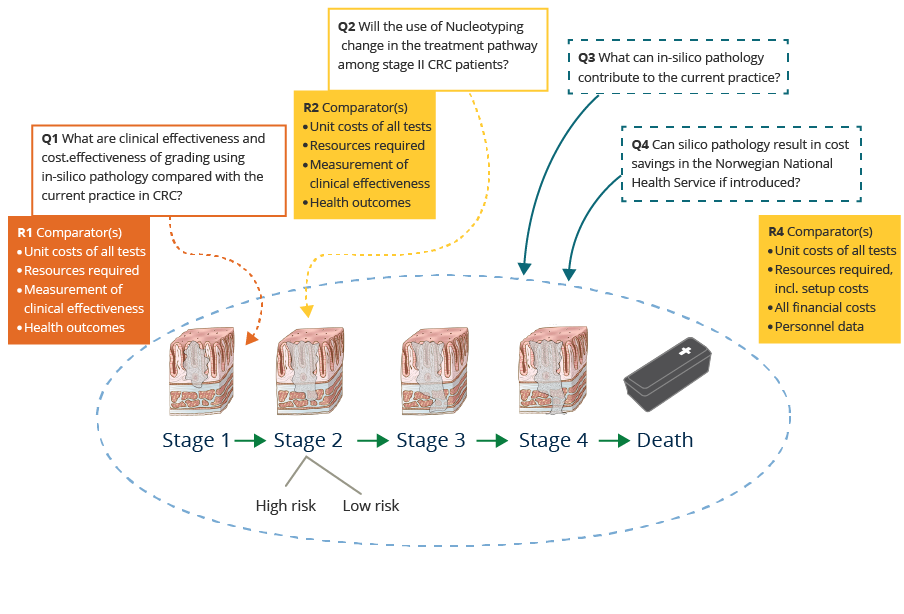 Summary of key questions/areas for discussion.
Summary of key questions/areas for discussion.
HEALTH ECONOMICS
There is limited literature on Health economics and cancer biomarkers. This is because it is challenging to conduct a periodic review of evidence and update of existing clinical guidance based on the best evidence. For example, the drugs Cetuximab and Panitumumab that use companion diagnostics have been evolved through further refinement of technologies leading to the identification of subgroups who would be benefitted from the technologies of interest (NICE, 2017).
Contrary to the rising expectations, there has been less enthusiasm about the research and development of biomarkers and personalized medicines in relation to the return of investments (ROI) for technology developers and the budget impact for healthcare payers. It is because the assessment of the pharmaceutical and companion diagnostic package can be undertaken in much the same way as for pharmaceuticals without companion diagnostics. However, in circumstances where alternative tests are available, for example, proprietary test kits or “in-house tests” for the same biomarker that would fulfil the requirements of the pharmaceutical marketing authorization, the amount of extra effort to fully evaluate these alternative options is likely to exceed the available resources and timeframe in technology appraisals (NICE 2013).
Defining the decision problem is the first step to be taken for the health economic evidence directly related to diagnostic biomarkers (Payne 2014; NICE 2011). This has been the main task so far. The following steps are to conceptualize the model: Select the relevant decision analytic model, build the decision analytic model, collect data to populate the decision analytic model, calculate expected costs and consequences, identify uncertainty and present the results.
The complete process is rather resource intensive. Based on the current evidence base of DoMore! project, Nucleotyping or Histotyping could be seen as one of two potential areas for the scoping (decision problems).
Oslo, 15th February 2019
Navigate to the other Work Package descriptions: